Introduction
The goal of this workshop, held in Tucson, Arizona, in July, 1997 and funded by the National Science Foundation (DEB 96 26813), was to bring together scientists (nematode systematists and ecologists, along with experts in other organismal groups that present difficulties for inventory and identification) to determine if a consensus on the methods for survey and inventory of nematodes could be reached. This document presents the consensus agreement.
Issues of science related to systematics, ecology, conservation biology, pest management, and biodiversity prospecting serve as the complementary rationales for conducting a nematode survey or inventory. Because nematodes are microscopic roundworms that inhabit a variety of opaque substrates, the taxonomically diverse species comprising a nematode assemblage are typically surveyed or inventoried together, which contrasts with the targeted survey or inventory of a selected taxonomic group. Nematodes being among the most numerous and species-rich animal taxa, they abound in all habitats on earth, with the exception of the planktonic, and represent a major constituent of earth’s biodiversity. Further, the nematode’s importance as a biological model is well established (Appendix 1). Nonetheless, they remain a neglected group and represent a profound lacuna in many studies of systematics and ecology.
A major reason for the neglect of nematodes concerns difficulties with their adequate characterization and identification. Their simple anatomical design lends itself to convergence and makes description of new species difficult. Few animal phyla have been so resistant to mastery; even the most seasoned nematode taxonomists are confident with only a restricted subset of the group. As a result, few organismal groups stand to benefit more significantly than nematodes from molecular techniques of characterization and delimitation of species.
The nature of species in any organismal group is a subject fraught with uncertainties. The Biological Species Concept (BSC) is commonly recognized as an acceptable conceptual framework for the delimitation of species among diploid organisms. However, the BSC does not acknowledge independent (though reproductively compatible) evolutionary lineages, such as those found in the Nematoda, nor can it account for the enormous array of reproductive strategies found among nematode lineages (Appendix 2). The theoretical and operational shortcomings of the BSC make its use irrelevant and often impossible for delimiting nematode species.
Recovering all of the evolutionary units that reflect the epistemologically real species in a given habitat can be confounded by the constraints inherent to conducting a survey or inventory. Principal investigators proposing to survey or inventory nematode species must address the issue of which species concept they will adopt and make explicit the operations they will use to most accurately delimit these species. These operations must be suited to the geographic/ecological scope of the study and to its time constraints. Further, they should not preclude the kinds of in-depth follow-up studies that a survey or inventory will inevitably generate.
A necessary first pass will assign nematodes to morphotypes, since in many and perhaps most cases the nematodes will be assignable to some taxonomic level (family, genus) but not to named species. Morphotypes should then be subjected to further analysis. The exact nature of the analysis may vary among studies, but principal investigators must rationalize their choice of molecular and/or morphological characters within the context of their species concept. Thus, the principal investigators will articulate the level of difference they accept as defining a species and justify this choice with reference to:
The species concept they advocate as most accurately recovering nematode species.
The operational ability of their molecular marker to delimit species as defined by the species concept advocated.
The goals and scale proposed for study.
The potential future use of data by the principal investigators and other researchers.
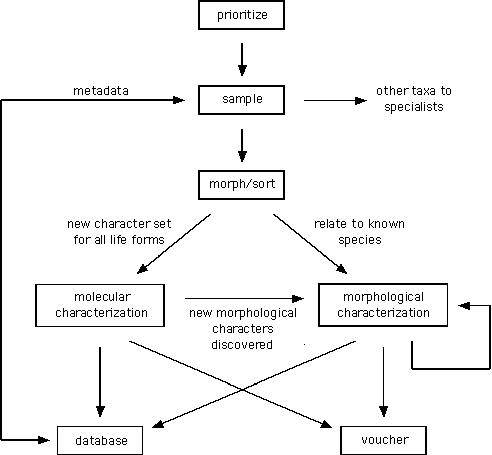
The tasks required to conduct a nematode survey or inventory are illustrated in Appendix 3, Fig. A3.1.
Discovery of new nematodes
The rationale for conducting a nematode survey or inventory should emphasize the discovery of new nematode species. The newly discovered nematodes may be important in different habitats for different reasons, and as a result, a survey or inventory involves the consideration of taxonomy, habitat, and scientific and economic importance. These issues are summarized below. Because methods of nematode extraction recover entire assemblages from soil, a survey or inventory will have to be focused to some degree, perhaps by emphasizing a taxonomic group, microhabitat, or ecosystem service. However, the focus of a survey or inventory should not result in discarding non-target nematodes. Provision should be made for the passing on of the nematodes that are outside the project’s immediate interests and expertise. This is essential to obtain maximum value of the survey or inventory.
Taxonomic considerations. We want to recognize nematode groups that are species-rich but under-represented in existing taxonomic collections, i.e., Enoplia, Chromadorida and Dorylaimida. An additional consideration is the discovery of significantly novel taxa representing new families or genera. However, any push to discover new nematodes must be tempered by extant taxonomic knowledge. The discovery process must be grounded in the reliable diagnosis of known species. Hence, new data may well need to be gathered on poorly described known species. In particular, molecular characterization must not be applied only to specimens from the surveyed target area, but also (to the degree possible) to relevant animals from type localities and/or reference cultures originally obtained from outside the target area.
Habitat considerations. Most non-agricultural habitats, including marine and freshwater habitats have been poorly sampled for nematode taxa and so are of high priority for the discovery of new species. Of related interest are ecologically important habitats, such as remnant native forests and habitats that are under threat of extensive human impact, such as freshwater streams, lakes and wetlands. Accordingly, geographic location should be considered when devising a sampling protocol: as a rule-of-thumb, widely-spaced samples from within a habitat-type have priority over those in close proximity.
Scientific and economic importance. The importance of discovering new nematode species involves issues of science and economics. Scientific measures of importance are often broad in scope, deal with long time scales and are difficult to quantify in monetary terms, whereas economic measures of importance are generally limited in scope, refer to relatively short time scales and are readily quantifiable. Our possible inability to quantify the immediate economic impact of discovering new nematode taxa does not indicate a lack of economic consequence, as recent calculations of the benefits of long-term “ecosystem services” have shown large dollar values. Nonetheless, the priority of discovering new taxa in different groups differs, depending on the relative weight given to the two ways of measuring importance, and this should be explicitly detailed. The plant-parasitic Tylenchida, insect-parasitic Rhabditida, and vertebrate-parasitic Strongylida and Ascaridida are examples of nematode orders that have clear economic importance (and of course these are also the best inventoried). In terms of ecosystem services and other long-term criteria, free-living nematodes from the other nematode orders have clear importance.
Sampling
When planning a nematode survey or inventory, protocols of sampling, extraction and specimen processing must result in specimens that are amenable to both morphologic and molecular analysis. Further, metadata collected at the time of sampling should accommodate the multiple uses of survey and inventory data.
The goal of a nematode survey or inventory is to capture information about the presence of nematode species occurring in one or more landscapes, ecosystems or geographic regions. Hence, the objectives and priorities of a survey or inventory determine the characteristics of an adopted sampling protocol, which ideally should represent an optimal mix of expertise, resources and time. Sampling details, such as substrate sampled, sample number, sample size, sample location and sample depth, warrant consideration when developing a protocol. However, the overriding issues of sampling pertain to sampling scale, pattern of sample collection and strategies of metadata collection.
Scale. The scalar dimensions of a survey or inventory relate to the breadth and depth of the sampling effort. Sampling breadth denotes the relative number of sites from which samples are collected, whereas sampling depth denotes the extent of taxonomic characterization per sampling site. A survey or inventory can rarely characterize a large number of taxa from a large number of sites. Apportioning resources between sampling breadth and depth entails a choice between an extensive or intensive sampling protocol. An extensive protocol maximizes the number of sampling sites and characterizes a limited number of taxa per site. An intensive protocol limits the total number of sampled sites but maximizes the number of characterizations per site. The rationale for conducting a survey or inventory will determine whether an intensive or extensive approach is more appropriate.
Collection. The next consideration in developing a sampling protocol is the spatial and temporal pattern of sample collection. When the principal investigator directing the survey or inventory retains control of the entire sampling process, the patterns of nematode distribution should take precedence over project logistics in developing the collection protocol. However, if the survey or inventory relies on the contributions from many cooperators, who might collect samples at different times over a wide geographic area, then logistics inevitably becomes the driving consideration in sample collection. A distributed collection pattern takes advantage of cooperator effort to collect samples based on broad sampling criteria. Such a strategy can generate new data at nominal expense and, for some purposes, results in information that satisfactorily addresses a pressing lack of knowledge about nematodes. More often, the rationale for conducting a nematode survey or inventory requires that samples be collected with greater rigor than is possible under a distributed plan.
Alternative strategies for the rigorous collection of samples include random, proportional, stratified and selected collection patterns. A random collection pattern assumes nematodes are uniformly distributed throughout sampled habitats. This assumption might be justified when nematode distributions are unknown or when a limited geographic area is being sampled. A proportional pattern apportions the collection of samples based on representation of habitats within sampling sites. The number of samples collected from a particular habitat might be proportional to the geographic extent of the habitat within a landscape. Similarly, a stratified pattern weights the importance of habitats, and then, highly weighted habitats are more intensively sampled than low-weighted habitats. Under a stratified collection plan, all habitats are sampled to varying extents, and the relative importance of different habitats is determined by the rationale governing the survey or inventory. A selected pattern of sample collection restricts sampling to a single habitat or a small number of habitats within the sampled sites.
Metadata. When developing a sampling protocol, accommodation should be made to facilitate the future use and interpretation of survey or inventory data by the inclusion of associated (meta)data. The kinds of metadata that might be collected include: geographic coordinates, characteristics of the vegetation, selected soil physical and chemical properties, and information about soil biota other than nematodes (which might require coordination of the sampling effort with specialists in other organismal groups). The challenge posed by metadata is to identify it, collect it, and archive it. These activities should represent an integral part of the nematode survey or inventory, thereby conferring greater value on the information about nematode species occurrences.
Morphologic characterization
A necessary first pass in any analysis will assign material to morphotypes. In many, and perhaps most, cases, material will not be assignable to named species. However, material can be identified to some taxonomic level (family, genus) and can subsequently be passed to appropriate experts and to laboratories where molecular analysis can be performed.
All surveys include a step in which nematodes are sorted into morphologically discrete units. These units may vary in taxonomic content due to limitations of morphological characters for delimiting nematode taxa. Morphological units may actually consist of assemblages of cryptic species or ecotypic variants of a species. It is essential that molecular-based approaches link the molecular phenotype to these morphological units. Photographic morphometric analysis and the maintenance of voucher specimens will reinforce the linkage complementation between morphological and molecular approaches.
Use of molecular approaches
Extraordinary species diversity, the paucity of trained nematode taxonomists, and the difficulty of using morphologic characters for species-level identification challenge nematode taxonomy. New approaches are needed to aid species identifications within the context of a classical morphological system. Without the ability to easily and efficiently discriminate among species, nematodes will remain excluded from many areas of biological research.
Taxonomic surveys of soil nematodes could be made much more efficient by the development of molecular techniques for species-level identification of individuals. The exact nature of the molecular analysis may vary from study to study. However, we identify here the major criteria with which to evaluate candidate systems. Whatever system is chosen, the principal investigator will have to address several issues outlined below and justify their choice with respect to the goals and scale of the proposed study and the potential for future use of their data by other researchers.
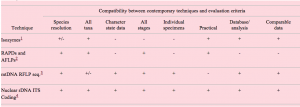
An ideal molecule for species identification should have several characteristics. Consideration of the following attributes will be important for the evaluation of proposed methods. An evaluation of contemporary techniques by each of these attributes is presented in Table 1.
Species-level resolution or diagnosabilty. The system must be able to discriminate species. The molecules chosen must evolve rapidly enough to display species-level differences; however, high levels of intraspecific variation will complicate species definitions.
Phylum-wide approach. A molecular approach that can be universally applied will be more useful for taxonomically diverse surveys than group-specific approaches.
Character state data. The data should be amenable to phylogenetic analysis. Ideally, such data should include clearly homologous characters which are useful in the broadest possible context. Character state data is important for the incorporation of cladistic species concepts. In addition, such data will contribute to the overall understanding of nematode relationships.
Identification from all species forms. In surveys and inventories, each species will usually be represented by different and/or multiple sexes, different forms of each sex (in more complex life cycles), eggs, and all juvenile stages including dauer juveniles. An accurate molecular method must identify all recovered stages of development.
Single nematode identification. The method employed must work on individual nematodes allowing for evaluation of intra-specific variation. Without prior knowledge of species-level identification, it is not possible to pool nematode samples for molecular analysis.
Practical for large-scale species-level taxonomy. The method should be practical, routine, and possess the potential for future automation. The far-reaching goal of a species-level taxonomy for nematodes is enormous and will ultimately require large-scale data collection and analysis.
Amenable to database/analysis. Methods which facilitate the collection of unambiguous and non-subjective communication of taxonomic characters are favorable. Data must be in a form that is useful to all nematode taxonomists.
Comparable datasets. The availability of comparable datasets for homologous molecules in other taxa will provide for useful comparisons.
Table 1. Evaluation of contemporary techniques for the assay of molecular markers capable of identifying and quantifying nematode genetic diversity.
Compatibility between contemporary techniques and evaluation criteria
1Isozymes are used widely for analysis of genetic diversity in many animal groups. Nevertheless, isozymes are often stage/sex specific, display high levels of polymorphism, and are not amenable to database analysis. The application of protein-level analysis for broad nematode taxonomy is not practical.
2RAPD amplifications can be obtained from individual nematodes. However, the reliability, consistency and reproducibility of RAPD-PCR using template derived from individuals is suspect. In addition, high levels of polymorphism, ambiguous homology, and difficulties for database analysis make the use of RAPDs ineffective for large-scale species-level identification.
3Due to the high levels of intraspecific variation and the lack of identified universal priming sites, the use of mitochondrial loci for species-level identification may be impractical.
4We suggest that sequences from both coding and non-coding regions of the nuclear rDNA repeating unit provide a useful starting point for species discrimination, although the efficacy of rDNA analysis may vary among the taxa under consideration.
Voucher specimens
The value of a biotic survey or inventory is measured by conservation and accessibility of its data. This includes data consistent with the immediate objectives, and data with added on value for ongoing and future complementary investigations. Specifically, when collecting nematodes, other nontarget taxa incidentally may be collected. Collection, curation and exchange of nontarget groups, including specimens and molecules, is particularly desirable if it maximizes data from limited collection resources. Such cooperative exchange is strongly encouraged. Conservation of data must include preparation and deposition of morphological voucher specimens by methods which maximize long-term preservation, curation, and availability in at least one major institutional collection (as recognized by the discipline) (Lichtenfels and Pritchard 1982, Robbins et al. 1992). When surveys or inventories include molecular data, provision should be made for appropriate linkage (i.e., databases) to morphological vouchers represented by either the actual specimen from which DNA is extracted, especially when that extraction is designed to minimize loss of morphological data, or additional specimens from the target population. When combined with morphological voucher specimens, photographs/drawings/and morphometrics derived from specimens prior to microdissection for molecular characters are important for linkage between morphological and molecular data. A particular survey or inventory must consider the potential and value for conserving a range of additional data including frozen tissue for molecular extraction, as well as frozen-living and active cultures.
Appendix 1. Caenorhabditis elegans as a biological model
The nematode, Caenorhabditis elegans, has become a preferred model of cell interaction (including neurobiology) in developmental biology; its automictic hermaphroditic style of reproduction makes it particularly convenient for developing isogenetic backgrounds against which genetic effects can be analyzed; the nematode genome is small and dense (there are few introns, and introns tend to be small) making molecular analysis more assailable. The nematode genome project (accessible through the Caenorhabditis elegans WWW Server) will make available sequences for potential use in other biological analyses. We therefore consider the time ripe for applying advances on this popular molecular and genetic model to ecological and evolutionary analysis of biodiversity. The purpose of this WWW document, in part, is to outline some of the important issues surrounding the use of molecular advances in describing and evaluating the important contribution of nematodes to the biodiversity of America’s ecosystems.
Appendix 2. Clonal species in nematodes
One of the classical problems with application of most theory-based species concepts to nematodes is the apparently widespread occurrence of parthenogenesis. Nematode taxa often contain morphologically similar “species” with different reproductive modes and varying patterns of gene flow. One taxon that has been extensively studied in this respect is the root knot nematode genus Meloidogyne: of 24 species investigated, four are amphimictic, seven are facultatively meiotic parthenogenetic, and 13 obligatorily mitotic parthenogenetic (Triantaphyllou 1985). Interestingly, males occur in most species (even in the obligatorily parthenogenetic species) but they can be very rare and do not necessarily contribute to gene flow. Observations on preserved material suggest similar patterns in many other taxa, but do not allow precise counts in the absence of cytological data. Another complicating factor is the occurrence of hermaphroditic lineages: some taxa contain mixtures of fully amphimictic, facultatively hermaphroditic and obligatorily hermaphroditic “species”. Obviously, crossing experiments between facultatively hermaphroditic populations are difficult to interpret in terms of reproductive isolation. And again, males can be rare and in some cases do not mate at all.
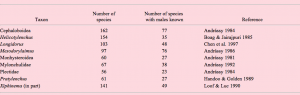
A relatively reliable indirect indicator of frequency of parthenogenesis is the presence or absence of sperm in the female reproductive system throughout populations. Unfortunately, this feature is not consistently mentioned in literature and can therefore not be quantified easily. Instead, as a VERY rough estimate of the presumed frequency of parthenogenesis in a diverse set of nematode taxa, Table A2.1 below gives an overview of the frequency of morphospecies for which males are known. Frequency of species with known males is definitely biased by sampling effort: small samples will easily miss rare males of facultatively parthenogenetic populations. Also, it does not in itself discriminate between parthenogenetic and hermaphroditic populations. For comparison, Table A2.2 lists two taxa known to contain hermaphroditic species. Finally, (as noted above) males can still occur in parthenogenetic lineages, but they are usually extremely rare in that case.
Table A2.1. Taxa known or presumed to contain amphimictic and parthenogenic “species.”
Table A2.2. Taxa known to contain amphimictic and hermaphroditic species.
Appendix 3. Operational tasks required to conduct a nematode survey or inventory
Figure A3.1. Schematic representation of a nematode survey or inventory showing the relationships among required tasks.
References
Andrássy, I. 1981. Revision of the order Monhysterida (Nematoda) inhabiting soil and inland waters. Opuscula Zoologica Budapest 17-18:13-47.
Andrássy, I. 1984. Klasse Nematoda. Bestimmungsbücher zur Bodenfauna Europas. Gustav Fischer Verlag, Stuttgart. 509 pp.
Andrássy, I. 1986. The genus Mesodorylaimus Andrássy, 1959 and its relatives (Nematoda: Dorylaimidae). Acta Zoologica Hungarica 32:207-261.
Andrássy, I. 1992. A taxonomic survey of the family Mylonchulidae (Nematoda). Opuscula Zoologica Budapest 25:11-35.
Boag, B., and M.S. Jairajpuri. 1985. Helicotylenchus scoticus n. sp. and a conspectus of the genus Helicotylenchus Steiner, 1945 (Tylenchida: Nematoda). Systematic Parasitology 7:47-58.
Chen, Q.W., D.J. Hooper, P.A.A. Loof, and J. Xu. 1997. A revised polytomous key for the identification of species of the genus Longidorus Micoletzky, 1922 (Nematoda: Dorylaimoidea). Fundamental and Applied Nematology 20:15-28.
Handoo, Z.A., and A.M. Golden. 1989. A key and diagnostic compendium of the species of the genus Pratylenchus Filipjev, 1936 (lesion nematodes). Journal of Nematology 21:202-218.
Lichtenfels, J.R. 1994. Presidential address. Journal of Parasitology 0:831-840.
Lichtenfels, J.R., and M.H. Pritchard, eds. 1982. A Guide to the Parasite Collections of the World. Special Publication of the American Society of Parasitologists. Lawrence, Kansas. 79 pp.
Loof, P.A.A., and M. Luc. 1990. A revised polytomous key for the identification of species of the genus Xiphinema Cobb, 1913 (Nematoda: Longidoridae) with exclusion of the X. americanum-group. Systematic Parasitology 16:35-66.
Robbins, R., V. Anderson, W.I. Decraemer, Z.A. Handoo, M. Mundo Ocampo, and E.M. Noffsinger. 1992. Why “voucher” specimens are important to you, the researcher. Nematology Newsletter 38:14-19. Note: Includes a list of voucher repositories.
Triantaphyllou, A.C. 1985. Cytogenetics, cytotaxonomy and phylogeny of root-knot nematodes. In Sasser, J.N., and C.C. Carter, eds. An advanced treatise on Meloidogyne, Volume I: Biology and Control. Department of Plant Pathology NCSU and USAID, Raleigh, North Carolina. 422 pp.